As all water users know, COD is an essential item in water quality monitoring. It means the amount of oxidant consumed when a water sample is treated with a strong oxidant under certain conditions. It is expressed in mg/L of oxygen. The chemical oxygen demand reflects the degree of contamination of water by reducing substances.
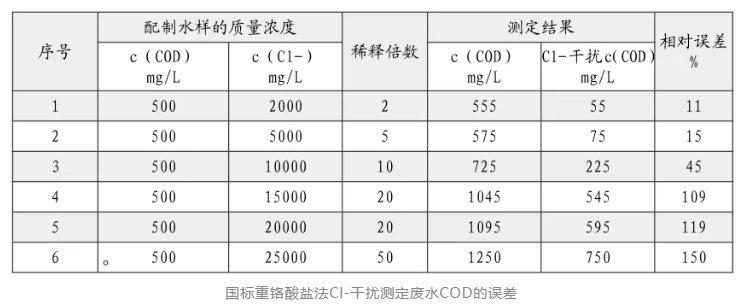
During the detection and analysis process, Cl- in the water sample is easily oxidized by the oxidant. A large amount of Cl- makes the measurement result high. The measurement of high-chlorine and low-COD wastewater is a difficult problem now.
In actual monitoring, it is found that many types of wastewater, such as chemical wastewater, monosodium glutamate wastewater, and seafood processing wastewater, have high Cl- contents. The COD measurement requires shielding of Cl- before measurement. Below, the editor will tell water users about the influence of Cl- and the method of eliminating Cl- interference.
Interference of CI- on COD determination
In GB11914-89 "Determination of Chemical Oxygen Demand of Water Quality - Potassium Dichromate Method", it is clearly pointed out that when the Cl- content in the water sample is too high, shielding agents such as mercuric sulfate need to be added to shield it. The influencing factors are mainly manifested in two points:
1. Cl- is oxidized by the oxidant, thus consuming the oxidant and causing the determination result to be high. The specific reaction equation is:
6Cl-+Cr2O72-+14H+→3Cl2+2Cr3-+7H2O
2. Silver salt is added to the reaction system as a catalyst. Cl- reacts with silver to form AgCl precipitation, which poisons the catalyst and affects the determination result.
Methods to eliminate CI- interference in COD determination
Dilution of water samples is one of the simple and effective methods. The national standard also mentions dilution of water samples with Cl- content exceeding 1000mg/L, but for water samples with high chlorine and low COD, too high a dilution multiple will affect the determination accuracy. At present, there are many methods to eliminate the interference of Cl-, mainly including mercury salt method, silver salt precipitation method, standard curve correction method, chlorine correction method, closed digestion method, low concentration oxidant method, KI-KMnO4 oxidation method, bismuth absorbent dechlorination method, etc.
Mercury salt method
The mercury salt method is also called mercuric sulfate complex method, which is the method of shielding Cl- in the national standard. That is, HgSO4 is used as a Cl-masking agent, and the mass ratio of HgSO4 to Cl- is preferably 10:1.
This method is very effective when the Cl-mass concentration is less than 200 mg/L, but when the Cl-concentration is very high, the measurement result is still high, and the error increases with the increase of Cl-concentration.
Because HgSO4 itself is highly toxic, and the mercury salt in the waste liquid is difficult to handle, and it will cause secondary pollution to the environment, many countries now do not advocate the use of this method to eliminate the interference of chloride ions, and are more inclined to seek non-toxic and pollution-free measurement methods.
Silver salt method
Silver salt precipitation method is a method of adding AgNO3 to generate AgCl precipitation to remove the influence of Cl-, which is suitable for water samples with Cl- mass concentration exceeding 10000 mg/L.
This method usually has two forms: one is to add AgNO3 during pretreatment and take the supernatant to determine the COD value. This method requires an appropriate amount of AgNO3 to completely precipitate Cl- and not excessive. The other method uses AgNO3 and KCr(SO4)2 as masking agents for Cl-. The role of KCr(SO4)2 is to inhibit the oxidation reaction of a small amount of Cl- during the digestion process.
The silver salt precipitation method uses precious silver salts, which increases the cost of determination, so it is necessary to recycle and reuse silver. Another disadvantage is that when AgCl precipitates, part of the organic matter in the water sample will be lost through coprecipitation and flocculation, making the determination result low.
Standard curve correction method
The steps of the standard curve correction method: first prepare a sodium chloride standard curve with different Cl- concentrations and determine the COD value, and draw a COD-Cl- standard curve. Then take two identical water samples, one for determining the COD value without masking Cl-, recorded as total COD, and the other for determining the chloride ion content. Find the corresponding COD value on the standard curve, recorded as COD·Cl-, and the difference between total COD and COD·Cl- is the true COD value of the sample.
The standard curve correction method does not use mercury salts and silver salts, is environmentally friendly and economical, and is the preferred method in the laboratory.
Chlorine correction method
During digestion, a reflux absorption device is used to export the generated Cl2 and absorb it with NaOH solution, and titrate it with Na2S2O3 standard solution. The amount of Na2S2O3 consumed is converted into the amount of oxygen consumed, which is the correction value of Cl-.
The actual wastewater COD value is the difference between the apparent value of COD and the correction value of Cl-. This method is suitable for the determination of high-chlorine wastewater with a chloride ion content of less than 20,000 mg/L and a COD greater than 30 mg/L. However, this method requires very careful experiments, otherwise it will bring more errors.
Sealed digestion method
The basic principle is to digest and determine COD in a closed container. When Cl- in the water is oxidized to Cl2 and reaches gas-liquid equilibrium, Cl- can no longer be oxidized. Using an appropriate masking agent, the COD value of the sample can be determined.
Compared with the standard method, the results of this method have higher accuracy and precision. Data show that the accuracy of COD analysis of high-chlorine wastewater using the sealed digestion method is high. When COD is between 100mg/L and 1000mg/L and the chloride ion concentration is less than 10000mg/L, the relative error of this method is ≤4.2%. Sealed digestion takes a short time, but this method has certain dangers, so the safety of the experiment must be ensured.
KI-KMnO4 oxidation method
Under alkaline conditions, KMnO4 is used to oxidize substances in the wastewater, the remaining KMnO4 is reduced with KI, and then titrated with Na2S2O3 standard solution, and the amount of Na2S2O3 consumed is converted into the amount of oxygen consumed, thereby obtaining a COD value.
However, the measured values of this method and the K2Cr2O7 oxidation method are different. There is a ratio K between the two. Therefore, you only need to know this ratio K to convert the COD value measured by the KI-KMnO4 oxidation method. This method is suitable for wastewater with Cl- content ranging from tens of thousands to hundreds of thousands of milligrams per liter, but it needs to measure the K value, so it is very cumbersome.
Bismuth absorbent dechlorination method
The Cl- in the water sample is released in the form of HCl gas in the acidic liquid, absorbed and removed by bismuth absorbent, and then the COD value is determined.
The accuracy and precision of this method are not significantly different from the standard method, but the digestion method is different, using microwave digestion or oven digestion.
The above-mentioned methods for eliminating Cl- interference have limitations in the actual experimental process, and each method needs further improvement. At present, when the Cl- concentration in the water sample is within 1000 mg/L, we give priority to using the national standard method; when the Cl- concentration exceeds 1000 mg/L and the COD concentration of the water sample is not high, we can consider using the standard curve method.